what classification of alcohol undergoes oxidation to yield a ketone
17.vii: Oxidation of Alcohols
- Page ID
- 36351
After completing this section, you lot should exist able to
- write an equation to represent the oxidation of an alcohol.
- identify the reagents that may be used to oxidize a given alcohol.
- identify the specific reagent that is used to oxidize main alcohols to aldehydes rather than to carboxylic acids.
- identify the product formed from the oxidation of a given alcohol with a specified oxidizing agent.
- place the alcohol needed to prepare a given aldehyde, ketone or carboxylic acid by uncomplicated oxidation.
- write a mechanism for the oxidation of an booze using a chromium(Six) reagent.
The reading mentions that pyridinium chlorochromate (PCC) is a milder version of chromic acid that is suitable for converting a primary alcohol into an aldehyde without oxidizing information technology all the style to a carboxylic acid. This reagent is being replaced in laboratories past Dess‑Martin periodinane (DMP), which has several practical advantages over PCC, such as producing higher yields and requiring less rigorous reaction atmospheric condition. DMP is named after Daniel Dess and James Martin, who developed it in 1983.

Oxidation States of Carbon
The general idea of oxidation and reduction reactions learned in general chemistry is that when a compound or atom is oxidized it loses electrons, and when it is reduced it gains electrons. In guild, to keep track of electrons in organic molecules a oxidation state ceremonial is used. Oxidation states to not represent the bodily charge but it will allow the number of electrons being gained or lost by a detail atom during a reaction.
To calculate the oxidation land of a carbon atom the following rules are used:
- A C-C bond does non affect the oxidation land of a carbon. And so a carbon attached to iv carbons has an oxidation land of zero.
- Every C-H bond will subtract the oxidation country of the carbon by 1.
- Each C-X bond will increase the oxidation state of the carbon by 1. Where X is an electronegative, such as nitrogen, oxygen, sulfur, or a halogen.
When looking at the oxidation states of carbon in the mutual functional groups shown below it can be said that carbon loses electron density as it becomes more than oxidized.
For this section, a simpler manner to consider this procedure is to say that when a carbon atom in an organic chemical compound loses a bail to hydrogen and gains a new bail to a oxygen information technology has been oxidized. A very commonly case is the oxidation of an alcohol to a ketone or aldehyde. Detect that during this process the carbon atom loses a hydrogen and gains a bond to oxygen.
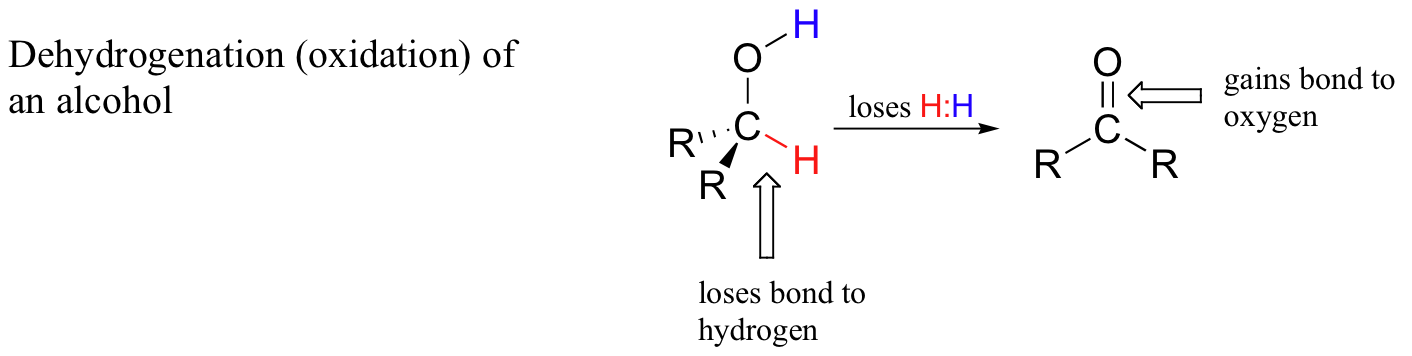
Oxidation of Alcohols
On of the nearly of import reactions of alcohols is their oxidation to carbonyl containing compounds such as aldehyde, ketones, and carboxylic acid. Typically primary alcohols, depending on the reagent used, produce aldehydes or carboxylic acids during oxidations. Secondary alcohols are oxidized to produce ketones, and tertiary alcohols are usually non affected by oxidations.
Alcohol Oxidizing Agents
Oxidation and reduction reactions ever occurs in tandem: when 1 compound is oxidized, another compound must exist reduced. For an alcohol to be oxidized in a reaction in that location must also be a chemical compound being reduced. This reduced compound is too chosen the oxidizing amanuensis. For example, chromium trioxide () is a common oxidizing agent used by organic chemists to oxidize a secondary alcohol to a ketone. During this reaction CrOiii is being reduced to . A common method for oxidizing secondary alcohols to ketones uses chromic acid (H2CrO4 ) as the oxidizing agent. Chromic acid, also known every bit Jones reagent, is prepared by adding chromium trioxide (CrOiii) to aqueous sulfuric acrid.

In that location is a wide selection of oxidizing agents bachelor for apply in the organic chemistry laboratory, each with its own particular properties and uses. In add-on to CrO3, other usually used oxidizing agents include potassium permanganate (KMnO4) and sodium dichromate (NatwoCr2O7). Any of these reagents tin can be used to oxidize secondary alcohols to form ketones and principal alcohols to form carboxylic acids. Tertiary alcohols remain unreactive to oxidation.
Full general Reactions
Case \(\PageIndex{1}\)
Oxidation of Benzyl Alcohol to Benzoic Acid
Oxidation of 2-Pentanol to 2-Pentanone
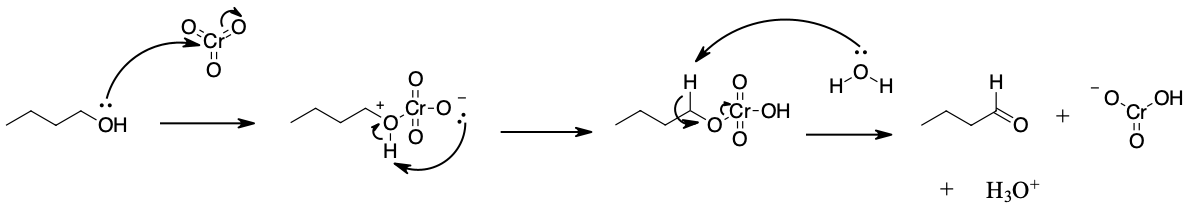
Mechanism
During this reaction mechanism the chromium atom is being reduced from Cr(VI) in the CrOiii starting material to Cr(IV) in the H2CrO3 product. Also, notice the the C=O bond is formed in the third pace of the mechanism through an E2 reaction. Although E2 reaction are generally know for forming C=C double bonds thought the emptying of a halide leaving group, in this case they are use to generate a C=O through the elimination of a reduced metallic every bit a leaving grouping.
Examples
Oxidation of aneo Alcohols with PCC to form Aldehydes
P yridinium chlorochromate (PCC) is a milder version of chromic acid. PCC oxidizes io alcohols one rung upwardly the oxidation ladder, turning principal alcohols into aldehydes and secondary alcohols into ketones. Unlike chromic acrid, PCC volition not oxidize aldehydes to carboxylic acids.Cr(4) as well as pyridinium chloride are produced as byproducts of this reaction.
Pyridinium chlorochromate (PCC)
General Reactions
Example \(\PageIndex{2}\)
Oxidation of 2-Phenylethanol to Phenylacetaldehyde
Oxidation of Cyclohexanol to Cyclohexanone
Mechanism
The first step of the mechanism is attack of alcohol oxygen on the chromium atom to course the Cr-O bail. Secondly, a proton on the (at present positive) OH is transferred to 1 of the oxygens of the chromium, peradventure through the intermediacy of the pyridinium common salt. A chloride ion is then displaced, in a reaction reminiscent of a 1,ii elimination reaction, to form what is known as a chromate ester.
The C-O double bond is formed when a base removes the proton on the carbon adjacent to the oxygen. Information technology is besides possible for pyridine to be used as the base of operations here, although but very low concentrations of the deprotonated form will be present nether these acidic atmospheric condition. In an E2 reaction, the electrons from the C-H bail move to form the C=O bond, and in the process break the O-Cr bond. During this footstep Cr(VI) gains 2 electrons to become Cr(IV) (fatigued here as O=Cr(OH)two).
Oxidation of oneo Alcohols with Dess‑Martin Periodinane (DMP) to form Aldehydes
PCC is being replaced in laboratories by Dess‑Martin periodinane (DMP) in dichloromethane solvent, which has several applied advantages over PCC, such as producing college yields and requiring less rigorous atmospheric condition (lower reaction temperature and a nonacidic medium). DMP is named afterward Daniel Dess and James Martin, who developed it in 1983.
Dess‑Martin periodinane (DMP)
General Reactions
Example \(\PageIndex{three}\)
Oxidation of Cyclohexanol to Cyclohexanone
Oxidation of Benzyl Alcohol to Benzaldehyde
Mechanism
The offset step of the machinery involves the reactant alcohol attacking the Iodine (5) atom and eliminating an acetate (Ac-) leaving group to form a periodinate intermediate. The next stride is a concerted E2-similar reaction where a hydrogen is removed from the alcohol, the C=O bond is formed, an acetate group is eliminated from the iodine atom, and the iodine (Five) atom gains ii electrons to be reduced to iodine (Iii).
Biological Booze Oxidations
In that location are many biological oxidations that convert a chief or secondary alcohol to a carbonyl chemical compound. These reactions cannot possibly involve the extreme pH conditions and vigorous inorganic oxidants used in typical laboratory oxidations. Rather, they occur at nearly neutral pH values and they all require enzymes every bit catalysts, which for these reactions usually are called dehydrogenases. An important group of biological oxidizing agents includes the pyridine nucleotides, of which nicotinamide adenine dinucleotide () is an example. This very complex molecule functions to accept hydride (H:-) or the equivalent (H++ 2e−) from the α carbon of an alcohol. The reduced class of NAD+ is abbreviated equally NADH and the H:- is added at the 4-position of the pyridine ring. Information technology is important to note that the hydride adds exclusively to the Re face of the pyridine ring giving NADH a pro-R stereochemistry.
An case of the remarkable specificity of this kind of redox organisation. One of the last steps in the metabolic breakup of glucose is the reduction of two-oxopropanoic (pyruvic) acid to L-2-hydroxypropanoic (lactic) acid. The reverse process is oxidation of L-lactic acid. The enzyme lactic acid d ehydrogenase catalyses this reaction, and information technology functions only with the L-enantiomer of lactic acrid. During this reaction a base of operations removes the alcohol hydrogen. The resulting alkoxide ion and so forms the C=O bail causing a hydride ion to transfer to NAD+.
The Construction of NAD+
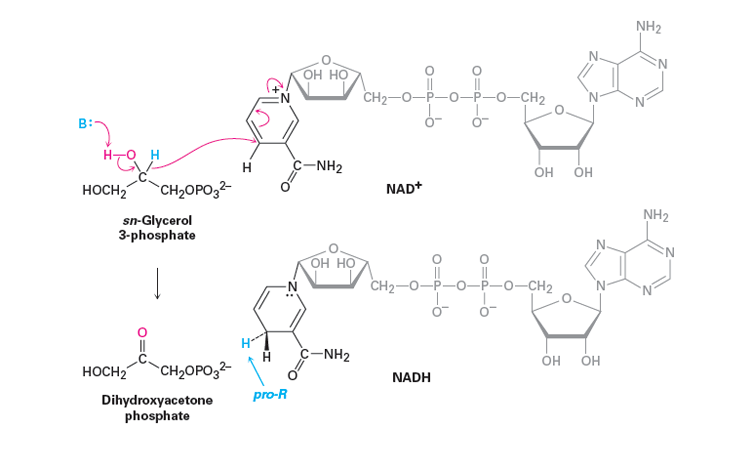
From an outside source. Convert mechanism to employ lactic acid.
Another example is provided past ane of the steps in metabolism by fashion of the Krebs citric acrid bicycle, is the oxidation of -ii-hydroxy-butanedioic (-malic) acrid to two-oxobutanedioic (oxaloacetic) acid. This enzyme functions but with -malic acid:
Exercises
Exercise \(\PageIndex{1}\)
Draw the alcohol that the following ketones/aldehydes would accept resulted from if oxidized. What oxidant could exist used?
- Answer
- 1)
-
a) Any oxidant capable of oxidizing an alcohol to a ketone would work, such as the Jones reagent (CrOiii, HtwoThen4, H2O), PCC, or Dess-Martin periodinane.
b) Since this is a primary alcohol, there are some precautions necessary to avoid germination of the carboxyllic acid. Milder oxidants such as the Dess-Martin periodinane, and also PCC (there is no water to class the carboxyllic acrid) would piece of work
c) Any oxidant capable of oxidizing an booze to a ketone would piece of work, such as the Jones reagent (CrO3, H2SO4, HtwoO), PCC, or Dess-Martin periodinane.
Show the products of the oxidation of 1-propanol and ii-propanol with chromic acid in aqueous solution.
- Answer
-
Show the products of the oxidation of one-propanol and 2-propanol with Dess-Martin periodinane.
- Answer
-
Source: https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Organic_Chemistry_(McMurry)/17%3A_Alcohols_and_Phenols/17.07%3A_Oxidation_of_Alcohols
0 Response to "what classification of alcohol undergoes oxidation to yield a ketone"
Post a Comment